Essential FAQs on Labeling in the Pharmaceutical Industry
Labeling is an essential yet often overlooked part of the pharmaceutical industry. It's not just about slapping a paper tag on a pill bottle. From drug facts to regulatory compliance, pharmaceutical labels carry a lot of weight. But what exactly goes into making these labels, and why are they so important? This practical FAQ guide has got you covered.
1. What is labeling in the pharmaceutical industry?
Labeling is an essential process in pharmaceutical manufacturing. It aims to provide critical information about a pharmaceutical product. The information on pharmaceutical labels is often presented in the form of printed texts or graphics.
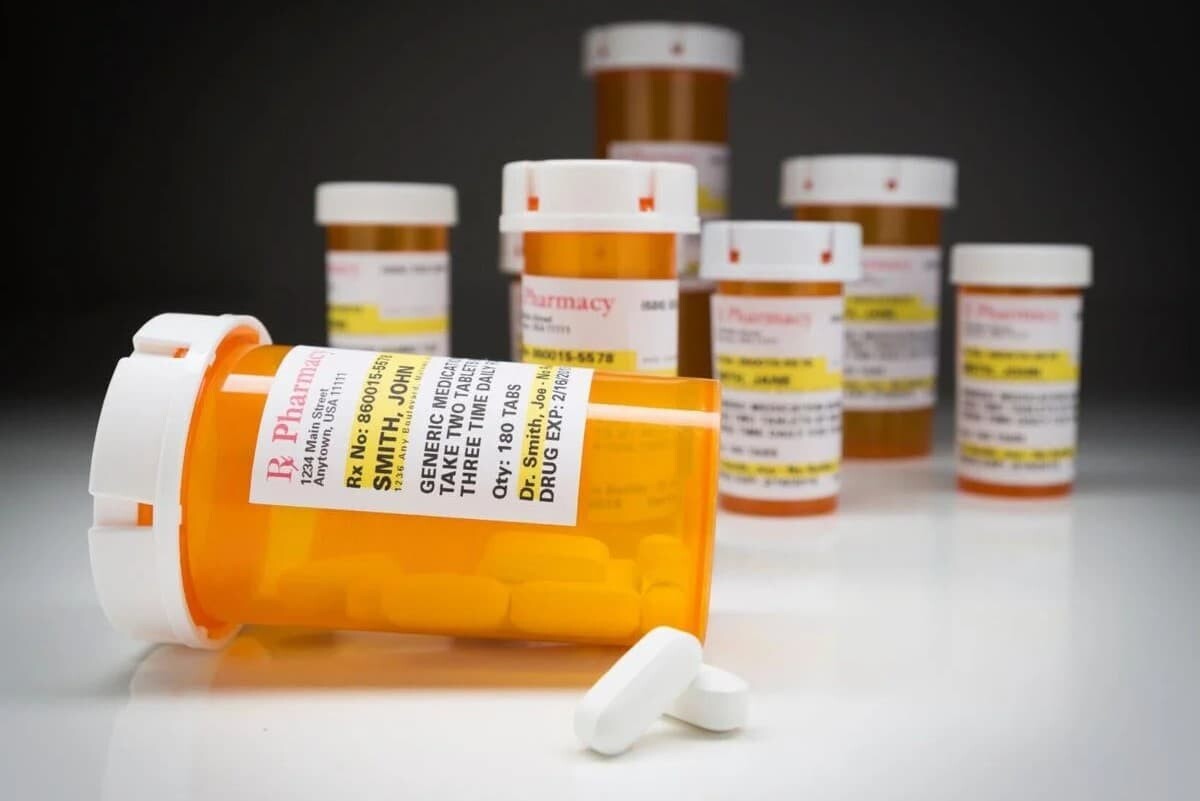
When you read the label, you can tell all the important details about the medication. For instance, you'll know what it is formulated with, how to use it, how much to take per day, and anything you should be aware of.
In simple terms, the purpose of labeling in the pharmaceutical industry is to ensure patients, pharmacists, and doctors use the medication in a safe and effective way.
2. Why is pharmaceutical labeling so important?
- Informed Use: Many drugs might look very similar. Labels are used to explain what they are specifically for. By reading labels, you'll know which one to choose for managing your symptoms.
- Patient Safety: Pharmaceutical labels guide you on how much to take and whether it should be taken with or without food. This information helps prevent overdoses or misuse.
- Risk Management: Labels warn you about possible side effects. This helps handle potential risks and avoid harmful reactions.
- Regulatory Compliance: Pharmaceutical products must meet strict standards because they can directly impact people's safety. Pharma companies are all required to adhere to stringent regulations about labeling. They must guarantee that their products have detailed and accurate labels.
- Storage Instructions: Most medications can be stored at room temperature, while some need to be refrigerated. So, the storage conditions are important. Labels often tell you where or how to store the drug to maintain its potency.
- Identification: In hospitals or pharmacies, numerous drugs need to be managed. Clear and accurate pharmaceutical labels help reduce the likelihood of errors.
- Traceability: A standard pharmaceutical label typically includes a barcode and serial numbers. These help track the medication from production to end use. The traceability of labeling is key for efficiently managing drug recalls. It helps manufacturers and regulatory bodies quickly identify and address issues with specific medication batches.
3. What are the different types of pharmaceutical labels?
Labeling in the pharmaceutical industry can be categorized into many different types.
Based on the Drug Type:
1. Over-the-counter (OTC) Drug Labels:
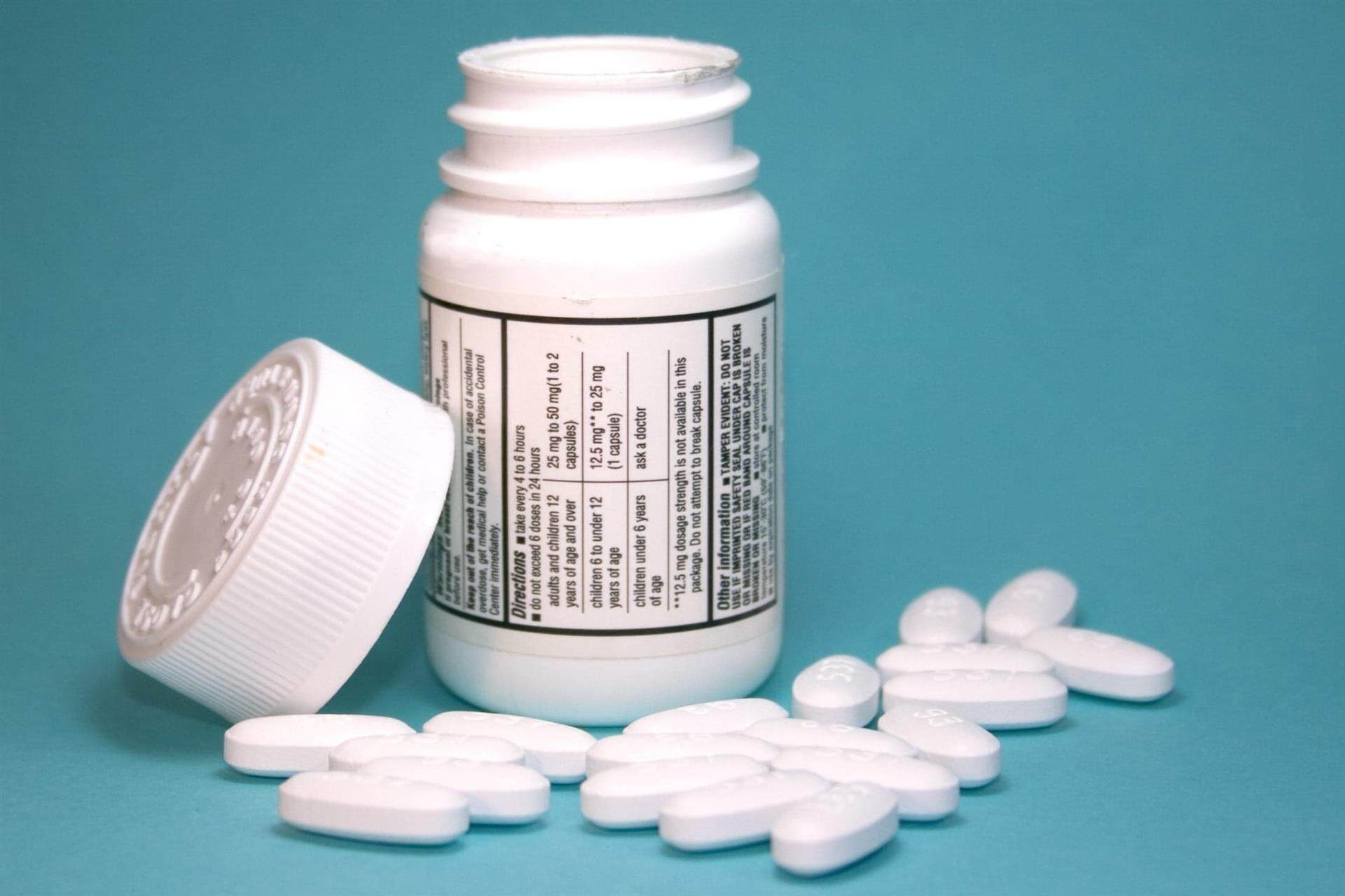
‐ For medications available without a prescription.
‐ Include standard information about the drug for consumers to use it safely on their own.
2. Prescription Drug Labels:
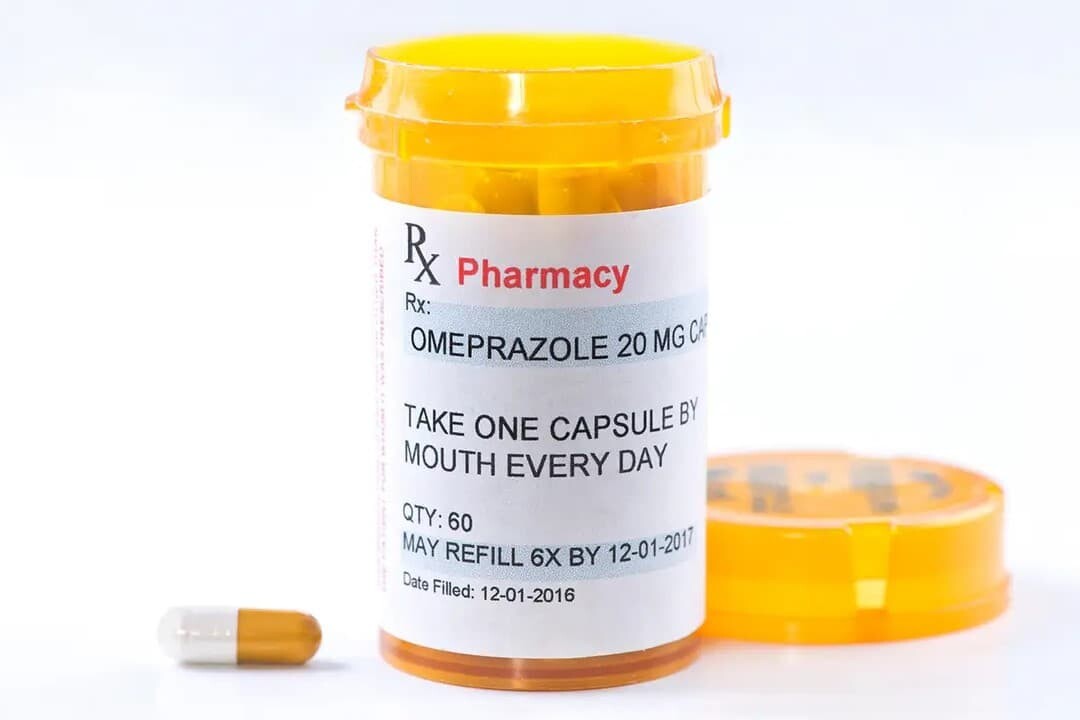
‐ For medications dispensed under a doctor's prescription.
‐ Include information about the patient, doctor, and pharmacy.
‐ Provide instructions tailored to the patient for safe and effective use of the medication.
Based on Labeling Purpose:
1. Primary Labels:
‐ Attached directly to the medication container.
‐ Provide essential information such as the drug name, dosage, and basic usage instructions.
2. Secondary Labels:
‐ Found on the outer packaging of the drug.
‐ Provide more detailed descriptions and instructions.
3. Auxiliary Labels:
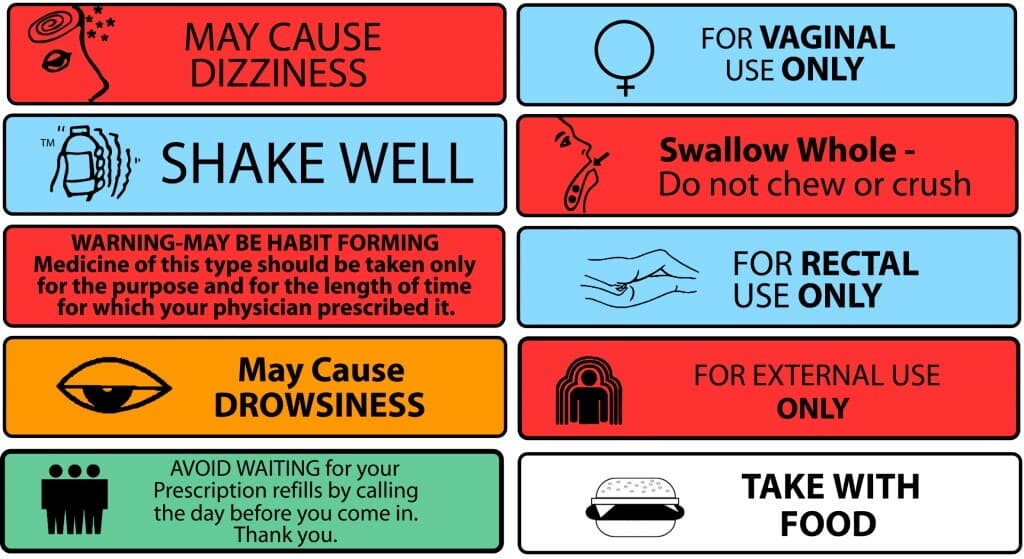
‐ Highlight the usage or warning, such as "May cause drowsiness," "Do not drink alcohol," "Shake before use," or "Take with food."
4. Tamper-Evident Labels

‐ Show if the drug has been opened before use.
‐ Ensure patient safety and maintain medication integrity.
5. Braille Labels

‐ Assist visually impaired patients in reading.
‐ Provide essential information about the medication in a tactile format.
6. E-labeling
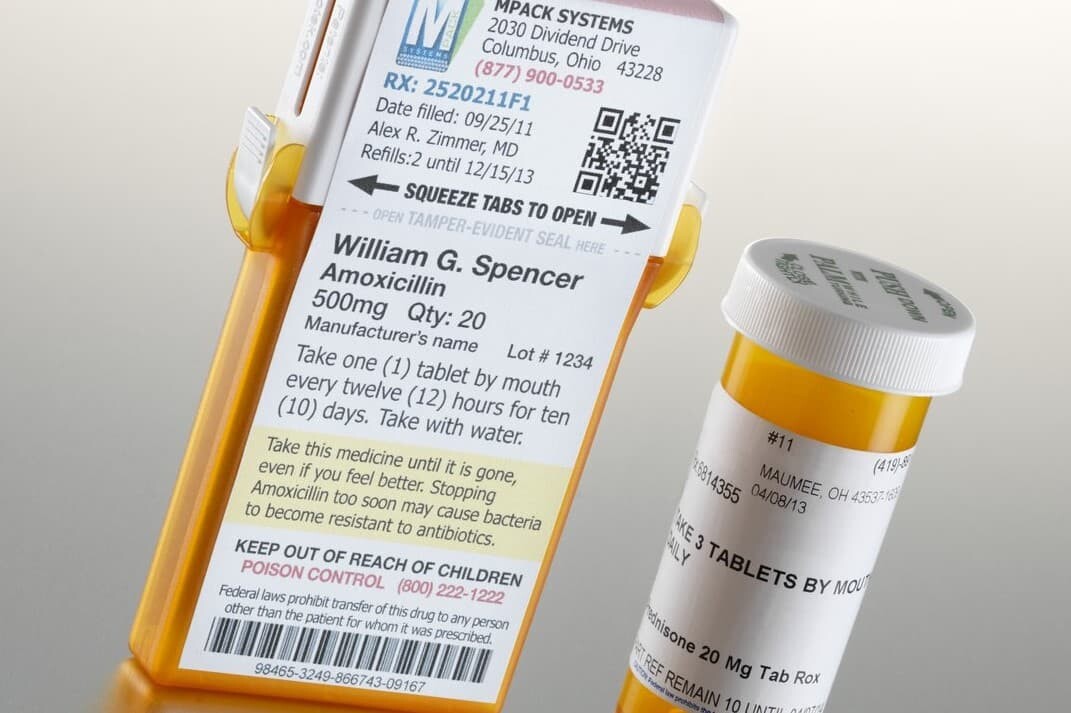
‐ Provide access to the drug information online through a QR Code.
‐ Offer more detailed, up-to-date information.
4. What information is typically required on pharmaceutical labels?
-
Over-the-Counter (OTC) Drug:
OTC drugs come with an easy-to-read Drug Facts Label. This includes:
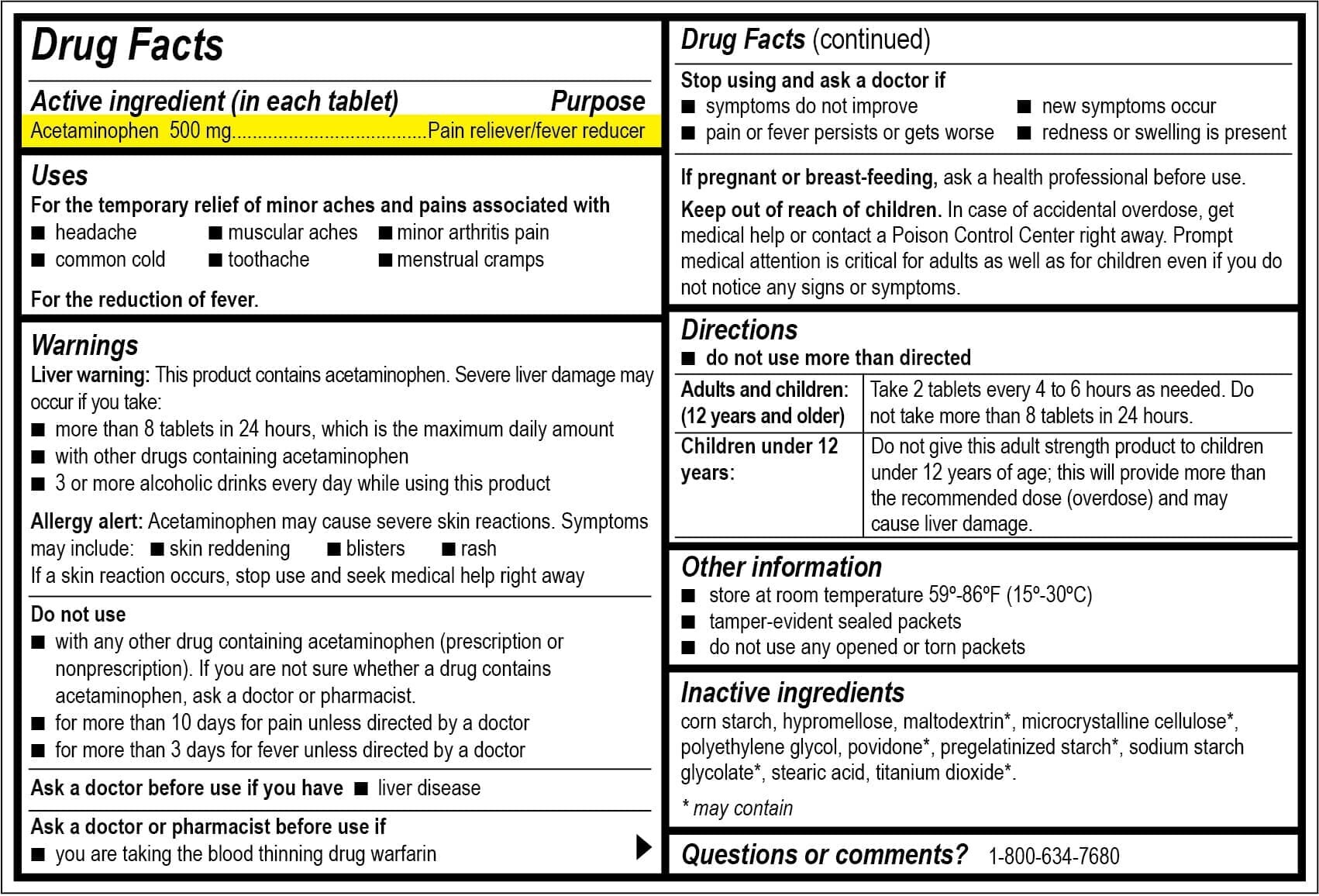
‐ Active Ingredient and Purpose: This section tells the chemicals in the medication and the amount in each dosage unit. Explain what the active ingredient will do.
‐ Uses: Describe the symptoms or conditions the medication treats.
‐ Warnings: Side effects, when not to use, and interactions with other drugs.
‐ Directions: This section shows you how much to take and how often.
‐ Other Information: How to store the medication and ingredients included or not included in the medication.
‐ Inactive Ingredients: Tell the chemicals that are not active but may cause allergy.
‐ Questions or Comments?: This section provides contact information for users if they need additional information about the medication.
Prescription Drug:
Labeling on prescription drugs is more complicated. It varies based on the conditions of individual patients. Still, there are some essential details.

‐ Patient Information: This includes the patient's name and address.
‐ Prescription Date: The date you received the prescription.
‐ Medication Name and Strength: The drug name and its dosage strength.
‐ Directions: Specific instructions on how and when to take the medication.
‐ Prescription Number: A unique number for the pharmacy to manage refills.
‐ Quantity, Refills, and Fill Date: Total number of pilled dispensed. The number of refills remaining. The date the prescription was filled.
‐ Pharmacy Information: Contact details of the pharmacy.
‐ Warnings: Important instructions or cautions, like "take with food" or "may cause dizziness."
‐ Expiration Date: Use the medication before the date.
5. How are pharmaceutical labels regulated?
Pharmaceutical labeling is not the same worldwide. It can vary based on countries or regions because they have their own regulatory bodies. Like the FDA in the United States or the EMA in the European Union, they set slightly different rules for what needs to be on a pharmaceutical label.
Here's a table that highlights the pharmaceutical labeling requirements in different countries and regions:
|
Aspect |
US (FDA) |
UK (MHRA) |
Canada (Health Canada) |
Europe (EMA) |
|
Regulatory Body |
Food and Drug Administration (FDA) |
Medicines and Healthcare products Regulatory Agency (MHRA) |
Health Canada |
European Medicines Agency (EMA) |
|
Primary Label Information |
Drug name, strength, dosage, route, active/inactive ingredients, warnings, expiry date, lot number |
Name, strength, route, batch number, expiry date, sterility, DIN, manufacturer/sponsor, specific warnings |
Brand name, proper/common name, dosage, expiry date, lot number, manufacturer, contact info |
Active ingredients, dosage form, strength, batch number, expiry date, legal status, marketing authorization number |
|
Language Requirements |
English |
English |
Bilingual (English and French) |
Official language(s) of the Member State, exemptions possible for certain products |
|
Braille |
Not required |
Required for drug name |
Not required |
Required for the product name |
|
Additional Requirements |
"Drug Facts" label for OTCs, boxed warnings, adverse reactions, specific usage instructions |
Adverse reactions, special handling instructions, storage conditions |
Contact information for reporting adverse effects, specific instructions, directions for use |
"Blue box" for country-specific info, symbols/pictograms, special conditions, and specific storage instructions |
|
Changes Notification |
Prior FDA approval required for most label changes |
MHRA notification required, specific changes may require approval |
Health Canada must be notified; specific changes may require approval |
EMA notification required for label changes; specific changes require approval |
|
Legibility and Format |
Specific format requirements, clear and legible text |
Clear, legible, understandable format, with warnings and storage instructions |
Clear and legible, with a minimum font size recommended |
Easily legible, clear, indelible text, consistent with the EU-approved summary of product characteristics |
|
Optional Information |
May include consumer contact info, barcode for inventory tracking |
May include non-statutory information that doesn't overshadow statutory details |
May include additional information provided it doesn't conflict with mandatory info |
Symbols or pictograms allowed for clarity, provided they are not promotional |
|
Local Representative |
Not specified |
Not specified |
Must include contact details for a person in Canada to report adverse reactions |
Local representative information may be included in the blue box |
6. How are pharmaceutical labels made?
Creating pharmaceutical labels involves a few key steps. Here's a quick rundown:
STEP 1. Gathering Information
First, all the necessary details, like the drug name, dosage, instructions, warnings, ingredients, and expiration date, are compiled. This content needs to comply with regulations and be approved by authorities.
STEP 2. Designing the Label
Designers ensure the label size fits the packaging of the medication. They decide the font size, color contrast, and where to place text and images to make sure everything is easy to read and understand.
STEP 3. Choosing Label Materials
The next step is selecting the required label materials. It depends on the specific needs of the pharmaceutical product. Common materials are paper, film, foils, and adhesives.
STEP 4. Printing the Labels
With the design ready and materials chosen, the labels are printed using specialized equipment. The goal is to make them durable and legible.
STEP 5. Quality Control
After printing, a thorough check is done to catch any errors or defects, ensuring the labels are perfect.
STEP 6. Cutting and Finishing
The labels are then cut to the right size, and any additional features like protective coatings or security elements are added.
STEP 7. Winding
Finally, the labels are wound onto rolls or placed onto sheets, depending on how they will be used or applied later.
7. What type of machinery is used to apply pharmaceutical labels?
Labeling in the pharmaceutical industry can be done using various types of machinery. Regardless of the pharmaceutical product you're producing, there is a labeling machine that is right for your specific needs.

If you're looking to label drugs packed in a round bottle, the MT-50M Semi-Automatic Labeling Machine is a solid choice. It's built with durable stainless steel and aluminum, handles a variety of bottle sizes, and uses hot stamp ribbons for clean, contamination-free labeling.
Do your products come with a variety of surface shapes? The T30114 Semi-Automatic Labeling Machine will be your best bet. This versatile machine features both manual and automatic labeling options. It can apply labels to flat, concave, and even curved surfaces. It uses a tamp-blow technique for precise application.
Are you handling large-volume pharmaceutical labeling? Take a look at the PBTB-160 Automatic Labeling Machine. User-friendly yet cutting-edge, this machine is the best fit for different production volumes and labeling needs. Plus, the PBTB-160 can be integrated into your existing packaging line to boost productivity.
Leave your comment
Also Offers


Containment Automatic Capsule Filling Machine SFK-703

Fully Automatic Dosator Capsule Filling Machine CZ-40

Our Team
As an expert in the pharmaceutical and pharmaceutical packaging industry, iPharMachine has provided solutions for hundreds of pharmaceutical and health product manufacturers for 17 years. By visiting customers, we get good reviews from our customers.
- info@ipharmachine.com
- English Español Deutsche







